The transitional period for MDR will end on 26 May 2021, the “Date of Application” (DoA) of the Regulation. From that date the MDR will apply fully , leaving 1 year for IVD solutions to comply to IVDR. Is your organization ready for this shift?
Main challenges:
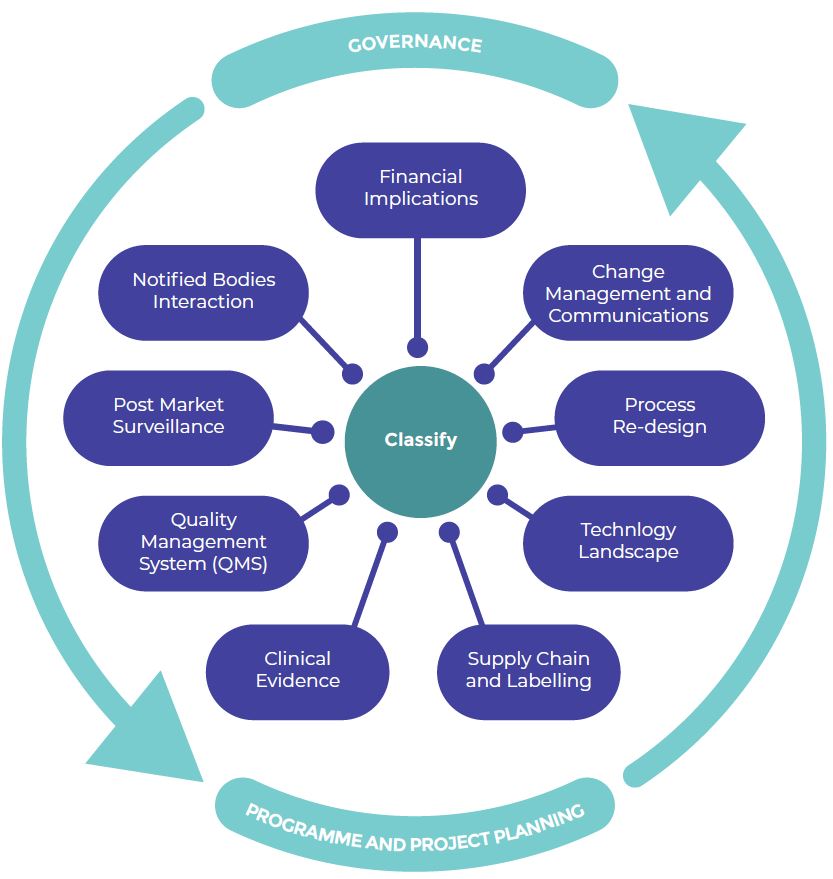
- Is my product portfolio touched by the new classification?
- Does the technical documentation and QMS system comply to the new rules?
- Are all economic operators involved in the product life cycle compliant and ready?
Receive your free roadmap
Receive your roadmap document by leaving your contact details below.

-
Veldkant 33A
B-2550 Kontich
Belgium -
Europalaan 28
5232 BC ’s-Hertogenbosch
The Netherlands

